
Using proteomics to improve the drug development process

Tyler Ford
February 23, 2023
Given that proteins imbue cells and tissues with their functions, it’s no wonder malfunctioning proteins are implicated as a cause of many ailments. Indeed, this is the reason why most drugs target proteins. Nonetheless, 90% of drug candidates fail clinical trials (Dowden and Munro 2019) and major reasons for failure include toxicity and a lack of efficacy (Sun et al 2022). Why? Drug candidates often impact proteins whose manipulation leads to detrimental effects on cell biology. Ultimately, targeting these proteins can lead to unacceptable levels of toxicity for patients. In this post, we outline the properties of a better drug target and highlight the ways comprehensive proteomics technologies can help drugmakers identify such targets.
Read our preprint to explore novel proteoform data and its potential in drug development.
View our animation to learn how proteomics can spur the development of precision medicines:
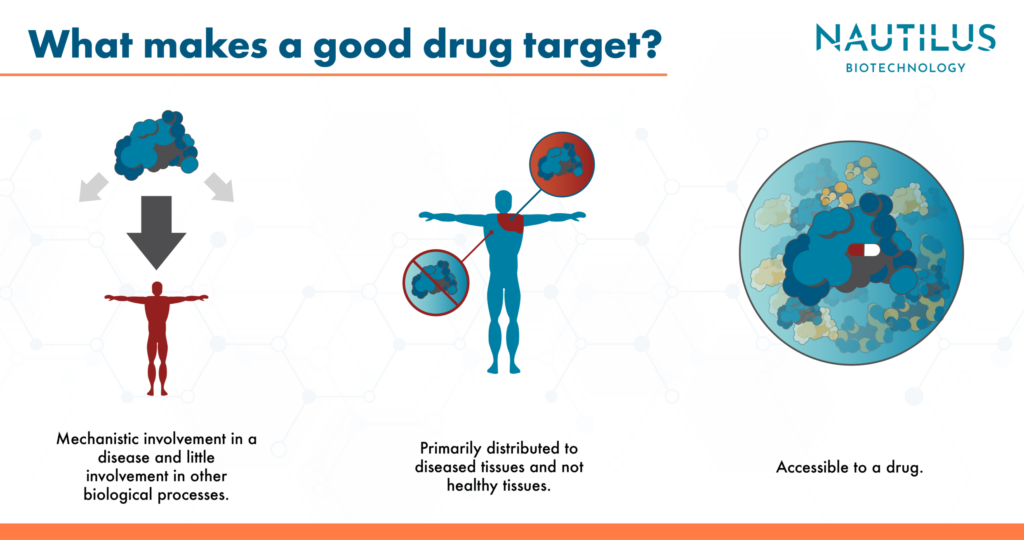
Properties of an ideal drug target
When researchers create drugs, they often design them to inhibit or activate a specific protein. To narrow research focus and potentially save time and money, proteins targeted by drugs should ideally have the properties described below. Importantly, biology is always complicated, and a drug target will have some mixture of these properties but will rarely be ideal. It is the essential job of the drug-maker to determine when risks associated with bad properties are low enough to confidently move forward with drug development.
- Mechanistic involvement in a disease – At a bare minimum, a drug target should somehow cause an ailment. That is, its function or malfunction should lead to the molecular events that cause a patient’s symptoms. Therefore, manipulating this protein should alter the symptoms. Ideally, the drug target will cause all the ill effects associated with a disease and not just a subset of them. In addition, it’s best if the protein is only involved with the negative impacts of the disease and nothing beneficial. Such ideal proteins will rarely exist, but targeting them should avoid unwanted “on-target” side effects.
- Protein distribution isolated to diseased cells or tissues – If a protein that causes a disease is only produced in a substantial amount in diseased cells or tissues, a drug developer can target it with more confidence that they won’t damage healthy tissues
- Accessibility and druggability – Some tissues are harder to reach with drugs than others (this is discussed as an important component of drug failure in Sun et al 2022). For example, it can be difficult to get drugs into the brain or into certain kinds of tumors. Ideally, a drug target will be easy to access, both in tissues and in individual cells. In addition, it should be feasible to create drug candidates that interact with the target protein. In other words, the target should be “druggable.”
Watch Nautilus Co-Founder and Chief Scientist Parag Mallick and Nautilus Senior Director of Scientific Affairs and Alliance Management Andreas Huhmer discuss how proteomics can expand the druggable universe:
Comprehensive proteomics studies can help researchers identify better drug targets
While traditional proteomics technologies are often difficult to use and only measure small fractions of the proteome at once, novel technologies are designed to be more sensitive, scalable, and easy-to-use. The Nautilus Proteome Analysis Platform in particular is designed to measure 95% of the human proteome at the single-molecule level. Some ways researchers can use such comprehensive proteome analysis for target validation during drug development include:
- Determining a drug target’s role in cellular functions and disease – Researchers have many means of genetically inhibiting or activating proteins before they design drugs to target them. These genetic experiments can reveal whether manipulating a protein causes a cell or animal model to manifest the symptoms of a disease. Nonetheless, with traditional proteomic techniques, it’s hard to get a holistic look at the ways altering a protein impacts biology. With the Nautilus Platform, researchers may be able to see whether manipulating a target specifically causes changes to protein biomarkers of disease, or whether proteins involved in other cellular functions are affected. If manipulating the drug target alters many cellular functions, targeting that protein could cause side effects that may ultimately lead to clinical failure.
- Measuring tissue distribution of potential protein targets – Side effects may manifest because a drug target is affected in both healthy and diseased cells. Prior to designing drugs against potential protein targets, researchers can measure the proteomes of healthy and diseased tissues to determine what druggable targets are most selectively produced in diseased tissues. Generally, a target protein that is more abundant in diseased cells than healthy cells may represent a better target.
- Determining intracellular distribution of a potential drug target – Proteomics technologies can also show researchers how druggable protein targets are distributed within diseased cells. For this, researchers can use various biochemical techniques to separate diseased cells into fractions containing different organelles and membranes, and measure the proteomes of these various fractions to determine where a series of potential targets resides. Then, developers can design their compounds to reach a drug target in a specific part of the cell.
- Measuring drug-protein interactions – Scientists can use some biochemical tricks like affinity purification to isolate proteins that bind to a drug candidate (reviewed in the context of mass spectrometry in Meissner et al 2022). Once isolated, next-generation proteomics platforms may be able to identify all these interacting proteins at the single-molecule level with high sensitivity. If a drug candidate interacts with many proteins and not just its intended target, it may be better to move on to a protein that can be targeted more specifically.
Improving drug development with proteomics
Creating a new drug is a long, complicated, and expensive process that leads to many failures as well as wasted time and money. We cannot maintain this status quo if we want to treat as many people as possible with affordable, accessible drugs, especially in the face of global crises like the COVID-19 pandemic. We are excited for researchers to use next-generation proteomics technologies like the Nautilus Proteome Analysis Platform to enhance the efficiency of the drug development process and get more affordable treatments to the patients who need them.
Watch this animation to discover what proteomics can teach us about cancer and cancer treatments:
MORE ARTICLES
