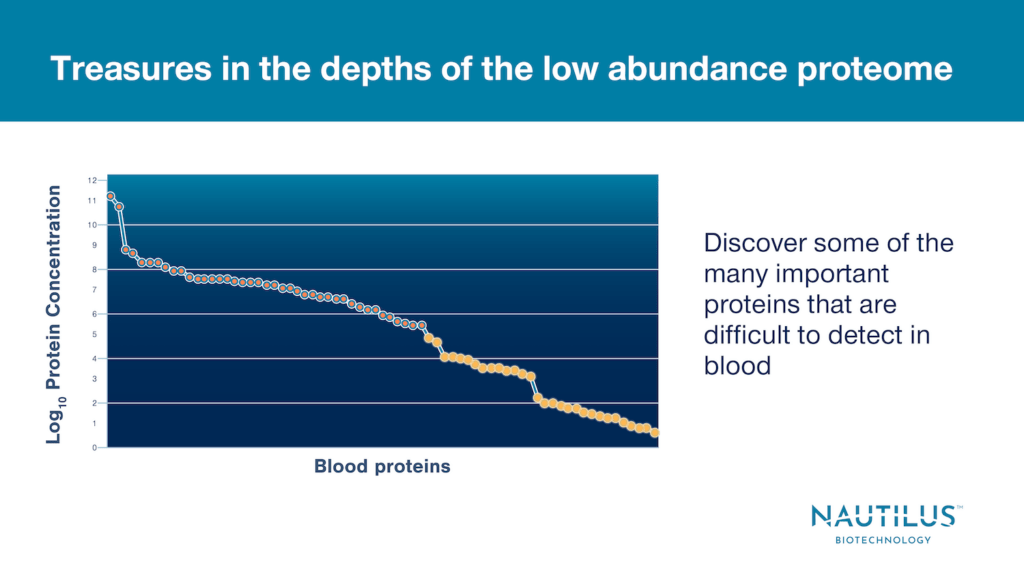
Protein levels fall across a wide dynamic range in the proteome. Some high abundance proteins make up a large fraction of the proteome, while low abundance proteins exist only in very small numbers. For example, protein abundances in the human blood proteome span 12 orders of magnitude.
In broadscale proteomics experiments it is critical to quantify proteins at both the high and low abundance ends of this range as both high and low abundance proteins can have impacts on biological function and cellular health.
For our purposes, low abundance proteins are those detected in small amounts compared to other proteins in a sample. There are often orders of magnitude fewer low abundance proteins, and they may be difficult to detect with current proteomics technologies.
In this blog post, we shine a light on some of the fascinating proteins observed at the low abundance end of the blood proteome (all proteins below are in the bottom 10% of the blood proteome as compiled by Deutsch et al 2021). While these proteins may not be measured consistently and precisely by current proteomics technologies, learning more about them as part of functional proteomics studies into the human proteome may reveal important biological insights. This post covers only a minuscule fraction of low abundance proteins in the human blood proteome.
The NautilusTM Proteome Analysis Platform is designed to enable researchers to uncover much more of this “dark proteome” in a consistent, precise, and comprehensive way. We hope proteomics platforms like ours will uncover the treasures hidden in the depths of the proteome and revolutionize the ways we understand health and disease.
Check out this animation to learn how the Nautilus Platform is designed to achieve high dynamic range
Chondromodulin-1 – A critical regulator of cartilage
The chondromodulin-1 protein is thought to play roles in cartilage development and maintenance as well cardiac valve maintenance, among other functions. Dysregulation of this low abundance protein appears to enable some forms of cancer to proliferate while inhibiting others. Researchers are even attempting to direct immunotherapeutic T-cells against chondromodulin-1 to fight Ewing sarcoma (a type of bone cancer).
It is not clear how chondromodulin-1 achieves its somewhat disparate effects in different cells. Comprehensive functional proteomics could help identify chondromodulin-1 interaction partners and better correlate chondromodulin-1 levels with its effects under different contexts. Such studies would reveal the specific scenarios under which this intriguing protein makes a good therapeutic target.
Bone morphogenetic protein 2 – Roles in bone development, maintenance, and beyond
Bone morphogenetic protein 2 was originally named for its ability to induce bone formation in soft tissues. Since then, researchers have discovered it plays a variety of roles in development (in bones and beyond). This protein continues to have many functions in adults and is particularly well-known for its ability to induce stem cells to differentiate into the osteoblasts and osteoclasts that maintain and repair bones. The FDA has even approved bone morphogenetic protein 2 to aid bone healing.
Despite decades of research into this protein, next-generation proteomics can reveal more about the complex patterns of its activity, including its relationship to other proteins in the human proteome, in a more holistic way. Such work could help refine models of signaling through bone morphogenetic protein 2-induced pathways and enable the development of drugs that precisely modulate these pathways. This may lead to better therapeutics for bone healing and more.
FGF23 – A phosphate regulating hormone with many other roles
Secreted by bone cells, the FGF23 protein plays a strong role in regulating phosphate levels throughout the body. Increased FGF23 in the bloodstream decreases phosphate uptake in numerous tissues including kidney tissues involved in phosphate reabsorption. Beyond its roles in phosphate regulation, FGF23 is believed to be involved in such varied activities as calcium retention, thyroid hormone production, vitamin D synthesis, and more.
Like many proteins in the human proteome, FGF23 comes in various proteoforms, and post-translational modifications modulate its activity. Its phosphorylation leads to degradation and the secretion of FGF23 fragments. FGF23 glycosylation, on the other hand, enables secretion of intact FGF23. Much is known about some aspects of intact FGF23 signaling, but there are still many gaps. It is also unclear what role FGF23 fragments play in signaling. Future targeted proteomics studies of this low abundance protein may help elucidate answers to these questions.
Overall, FGF23 is an incredibly important hormone. Comprehensive functional proteomics can provide more holistic views into the complex associations between FGF23 and the many biological functions it impacts. Such studies should help unravel the tangled web of interactions between FGF23, its fragments, and cellular signaling. This work may highlight important points of therapeutic intervention and help alleviate defects in phosphate metabolism, calcium metabolism, vitamin D metabolism, and more.
A trove of low-abundance proteins in the dark proteome
There are numerous understudied proteins that form the “dark proteome.” Many of these live in the depths of the low-abundance blood proteome and have potential links to disease. A few illustrative examples of these rare blood proteins include:
- TIPRL – A protein implicated as a potential biomarker of aggressive liver cancer.
- VAV2 – A protein involved in a variety of biological processes and recently identified as a mediator of cancer radiotherapy resistance
- Repetin – A calcium binding protein with decreased blood proteome abundance in patients with schizophrenia and bipolar disorder.
There is a great disparity between the number of studies on proteins like these (searching their names in PubMed delivers results numbering in the 10s to 100s) versus highly studied proteins like tumor suppressor p53 (thousands of publications). While one-off papers like those linked above point to the potential importance of the dark proteome, comprehensive proteomics studies will begin to efficiently reveal the many roles these and other understudied proteins play in all manner of healthy functions and disease.
Next-generation proteomics tools for exploring the dark proteome
These proteins may have been difficult to see in the past, but novel proteomics technologies will uncover them, while also enabling functional proteomics studies that show how they interact with the many other proteins across the wide dynamic range of the human proteome.
Only by gaining a comprehensive understanding of these proteins and their many interactions through next-generation proteomics technologies like the NautilusTM Proteome Analysis Platform can we truly understand the human proteome. Such platforms will enable researchers to develop effective ways to therapeutically target biological processes involving low abundance proteins like these and hopefully improve health outcomes for many people.
Checkout this video to learn how the Nautilus Platform is designed to quantify proteins
MORE ARTICLES


