Tau proteoforms as potential drivers of precision medicine – Applications of proteomics in neuroscience

Tyler Ford
January 30, 2025
Alzheimer’s disease is generally quite difficult to diagnose. Until recently, definitive Alzheimer’s diagnoses were only established postmortem through the identification of aggregates in the brain – insoluble amyloid plaques seeded by amyloid beta and insoluble neurofibrillary tangles (NFTs) seeded by the tau protein. Now, researchers are establishing means of diagnosing Alzheimer’s through the visualization of plaques and tangles with positron emission tomography (PET) and the detection of certain forms of amyloid beta and tau in the blood (see Palmqvist et al., 2024 for example). However, PET is not widely available, and it is not clear if blood biomarkers are specific to Alzheimer’s disease as opposed to other forms of dementia. For instance, tau blood biomarkers may be shared amongst diseases involving tau (“tauopathies”). These diseases may have similar effects on cognition yet different molecular causes.
To effectively diagnose and treat Alzheimer’s disease as well as other tauopathies, it is critical to gain a better understanding of their molecular nature. This may make it easier to use precision medicines to treat tauopathies and attack them at their molecular roots.
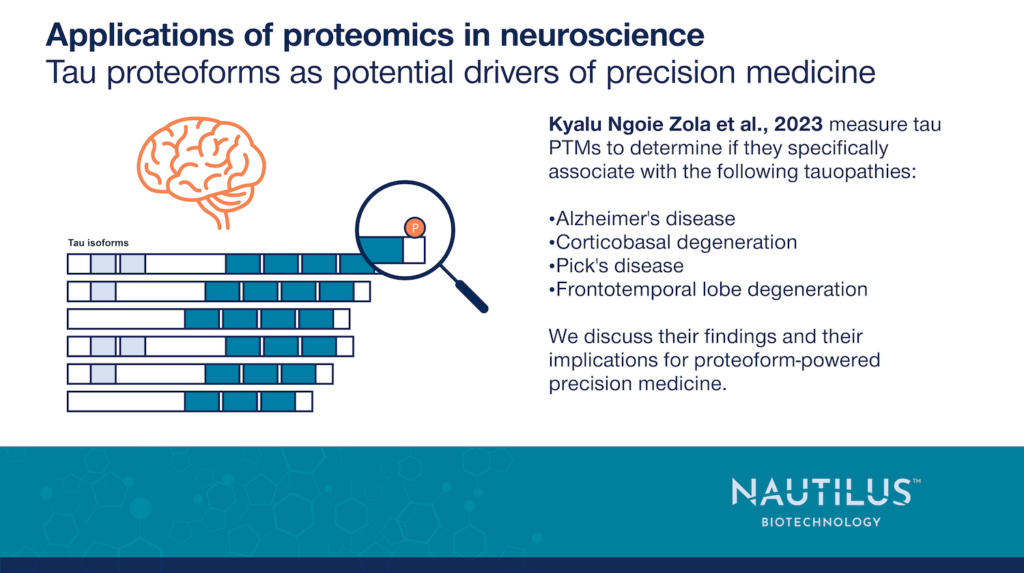
Nonetheless, researchers are already seeing clues that patterns of modification on specific tau isoforms may be indicative of AD progression (Wessling et al., 2020). In the work summarized below, Kyalu Ngoie Zola et al., 2023 show how particular tau PTMs and patterns of PTMs on tau peptides may make effective biomarkers that help diagnose Alzheimer’s and other tauopathies. Furthermore, these authors suggest how their data may point to the molecular mechanisms underlying tauopathies.
Find additional posts on proteoforms and Alzheimer’s here
The potential importance of analyzing soluble tau as opposed to insoluble tau
Prior to this work, Wesseling et al., 2020 did extensive work cataloging insoluble tau PTMs and isoforms present in post-mortem brain tissue extracts from Alzheimer’s patients with varying degrees of Alzheimer’s severity (see our blog post on this work for more information). Insoluble tau forms aggregates classically associated with Alzheimer’s disease. Wessling et al., 2020 showed that 4R tau isoforms as well as increased tau PTMs are found in insoluble tau fractions and are associated with disease progression.
In contrast, Kyalu Ngoie Zola et al., 2023 focused on soluble tau isolated from postmortem brain tissues of patients with various tauopathies and not just Alzheimer’s. They did this for two main reasons:
- They thought soluble tau from brain tissue might be more like tau found in cerebral spinal fluid (CSF) which can be relatively readily analyzed when drawn from live patients. Although insoluble tau forms aggregates, soluble tau may still have modifications indicative of Alzheimer’s disease. Thus, investigating soluble tau may point to more clinically significant tau biomarkers than insoluble tau.
- They wanted to determine if there were differences in soluble tau form different tauopathies. If so, they could use these differences to develop biomarkers that distinguish between the tauopathies.
Watch Nautilus Senior Director of Scientific Affairs and Alliance Management Andreas Huhmer discuss a new era of Alzheimer’s research with Sarah DeVos Ph.D. of Curie.Bio
Soluble tau extracts from various tauopathies have minimal differences in tau isoform levels but significant differences in tau PTMs
Kyalu Ngoie Zola et al., 2023 investigated differences between soluble and insoluble tau extracted from post-mortem brain tissues from patients with the following tauopathies:
- Alzheimer’s disease (AD) (n = 15)
- Corticobasal degeneration (CBD) (n = 5)
- Pick’s disease (PiD) (n = 5)
- Frontotemporal lob degeneration (FTLD) (n = 10)
While there were clear differences between the relative levels of insoluble 3R and 4R tau isoforms extracted from these samples, there were far less prevalent differences in the soluble isoforms.
When the researchers then used LC-MS to quantify modifications on tau peptides, they observed significant differences between soluble tau in the different diseases. For example, K317 was ubiquitylated in AD but not the other diseases. Similarly, peptides containing both Ub-K267 and P-S262 were also specific to Alzheimer’s disease.
Patterns of modification to soluble and insoluble tau suggest impacts on tau aggregation
Kyalu Ngoie Zola et al., 2023 used LC-MS to identify tau PTMs, and in their workflow, tau was enzymatically digested before analysis. The resulting examination of tau peptides made it impossible to determine all the PTMs and other alterations found together on intact tau protein molecules (i.e. it was impossible to identify tau proteoforms). Nonetheless, these researchers did identify tau peptides with multiple modifications and their observations suggest certain modifications may have aggregation-preventing effects.
For example, any soluble tau peptides that had P-T217 also had P-T212 whereas peptides with only P-T217 were observed in insoluble tau aggregates. The authors suggest that such associations may indicate that modifications like P-T212 (found only in soluble tau) prevent aggregation. They observed similar associations for a few other PTM patterns as well.
A need for studies with proteoform resolution
These findings are important because they point to the need to study patterns of modification as opposed to individual PTMs. Even with low resolution views of peptides, these authors have hints that combinations of modifications may be associated with specific processes and outcomes. With full proteoform resolution, researchers may resolve many additional, similar associations. Much more work must be done to determine how the patterns identified here are associated with tau aggregation, but such associations should be decipherable with new, proteoform-aware technologies.
Furthermore, studies like this one suggest that many of the molecular details of disease are obscured because researchers don’t have enough resolution in their views of proteins. Here PTM and isoform resolution provide clues to associations with specific diseases and disease processes, but these are difficult to interpret because they are established through the analysis of peptides. With bona fide proteoform resolution, researchers may be able to determine exactly how proteins are modified in diseases, determine how those modifications change protein structure/function in combination, and how interactions between proteoforms impact disease. Specific proteoforms may then be used as biomarkers for disease processes or targeted with novel treatments. Even if these treatments target the same protein, they may be made disease-specific through proteoform targeting and thus advance us to a new era of proteoform-enabled precision-medicine.
MORE ARTICLES