Tau proteoforms as potential drivers of cognitive decline – Applications of proteomics in neuroscience

Tyler Ford
March 20, 2025
The classic pathological features of Alzheimer’s disease are amyloid beta aggregates outside neurons (amyloid plaques) and tau aggregates inside neurons (neurofibrillary tangles or NFTs). Plaques have been primary therapeutic targets, yet immunotherapies successfully eliminating them slow but do not stop cognitive decline (Golde and Levey 2023, our recent blog post on amyloid beta proteoforms). These immunotherapies are powerful advances but not cures. For many, the next step in the fight against Alzheimer’s is a renewed focus on tau.
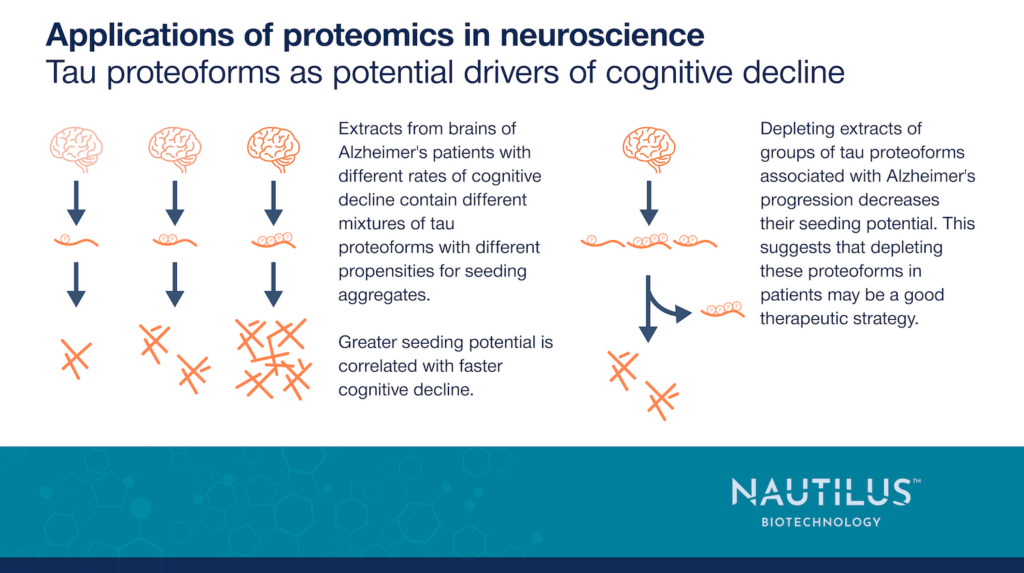
Tau has many molecular variants or proteoforms. It comes in 6 isoforms in the central nervous system, and these can be modified in various ways including phosphorylation, acetylation, and ubiquitination to produce a wide variety of proteoforms (Reviewed in Alquezar et al., 2021). In the work highlighted below, Dujardin et al., 2020 provided evidence that tau proteins extracted from Alzheimer’s patients with varying rates of cognitive decline have heterogenous proteoform profiles. These are associated with varying propensities to seed aggregate formation, and higher seeding potential is correlated with faster rates of cognitive decline. Finally, these authors showed that depleting brain extracts of groups of tau proteoforms with shared epitopes lowers their seeding potential. This work suggests depleting tau proteoforms associated with seeding may be a promising therapeutic avenue.
Tau seeding potential is correlated with cognitive decline
Dujardin et al. extracted proteins from the brains of 32 Alzheimer’s patients with varying levels of cognitive decline. Then, they tested each extract’s propensity to seed tau aggregation in a variety of models:
- An in-vitro FRET biosensor – HEK293 cells expressing tau proteins fused to fluorescent proteins were treated with extracts from patients. Increased FRET signals resulting from treatment indicated seeding. Extracts from different patients had highly variable seeding by this assay.
- Mouse primary neurons – Mouse primary neurons expressing human tau were cultured and treated with the Alzheimer’s brain extracts. Staining with antibodies that detect hyperphosphorylated tau – an indicator of aggregation – showed extracts that induced more hyperphosphorylation also had greater seeding potential in the FRET assay.
- Live mice – Patient extracts were injected into the brains of live mice expressing human tau isoform 1N4R. Two months after injection, the mouse brains were assessed for tau aggregate formation using an antibody that detects hyperphosphorylated tau. Hyperphosphorylated tau was more prevalent in brains injected with extracts that had high seeding potential according to the FRET assay.
These results showed extracts from different patients had varied seeding potential. Dujardin et al. went on to show there was a positive correlation between seeding potential and rate of cognitive decline. These intriguing results suggest seeding may be an important factor in disease progression.
Watch Nautilus Senior Director of Scientific Affairs and Alliance Management Andreas Huhmer discuss a new ear in Alzheimer’s research with Sarah DeVos Ph.D. of Curie.bio
Groups of tau proteoforms are associated with seeding potential
Knowing tau is heavily modified during Alzheimer’s disease progression (Wesseling et al., 2021), Dujardin et al. assessed whether seeding and cognitive decline were associated with groups of tau proteoforms defined by their higher order structures, post-translational modifications, or patterns of modification. They discovered that extracts with higher seeding potential also had:
- More oligomeric tau
- More hyperphosphorylated tau
- More high molecular weight tau
Bottom-up mass spectrometric analysis later revealed that some single phosphorylation events (e.g. pSer262) and some double phosphorylation events (E.g. pThr321 and pSer235) were positively correlated with seeding potential and cognitive decline while others were negatively correlated with seeding and cognitive decline.
Highlighting the need for better resolution of tau proteoforms, some single phosphorylation events within clusters of potential phosphosites were negatively correlated with seeding while the simultaneous phosphorylation of multiple sites within these clusters was positively associated with seeding. It can be difficult to determine which sites in a cluster are phosphorylated using bottom-up mass spec, but these results show that patterns of phosphorylation found on specific tau proteoforms or groups of tau proteoforms may help control seeding potential. Technologies that are capable of more fully identifying tau proteoforms and disambiguating their patterns of modification may reveal the links between these specific proteoforms and their pathological effects.
Targeting tau proteoforms as a potential path for precision medicines against Alzheimer’s
Given the association between groups of tau proteoforms, seeding, and cognitive decline, Dujardin et al. suggested that depleting these proteoforms may make a viable strategy for the development of precision medicines against Alzheimer’s disease. To test this concept, the authors depleted various groups of tau proteoforms from patient extracts using antibodies targeting tau epitopes and phospho-epitopes. Depletion of different epitopes had different effects on seeding depending upon which extracts and which epitopes were used. This may be anticipated given the heterogenous proteoform make-up in each patient. Nonetheless, depletion of proteoform groups known to be associated with Alzheimer’s progression was a consistently effective strategy for attenuating seeding, suggesting this approach has therapeutic promise.
A future for tau-targeted precision medicines
Taken together, these results indicate that particular tau proteoforms or groups of proteoforms are associated with Alzheimer’s progression. In addition, individual Alzheimer’s patients likely have distinct proteoform profiles contributing to their progression. By developing technologies that can resolve these profiles, it may be possible to effectively target and deplete a given patient’s specific set of pathological tau proteoforms. Such treatments may make effective precision medicines for Alzheimer’s disease.
MORE ARTICLES