At recent HUPO conferences, Nautilus team members have seen growing interest in proteoforms—molecular variants of proteins that drive biological processes. Research is only just beginning to reveal their impact, but mounting evidence strongly suggests proteoforms play a prominent role in Alzheimer’s disease. To highlight this, we’ve published a series of blog posts exploring how tau and amyloid beta proteoforms are linked to Alzheimer’s pathology and progression. Summaries and links to each blog post can be found below.
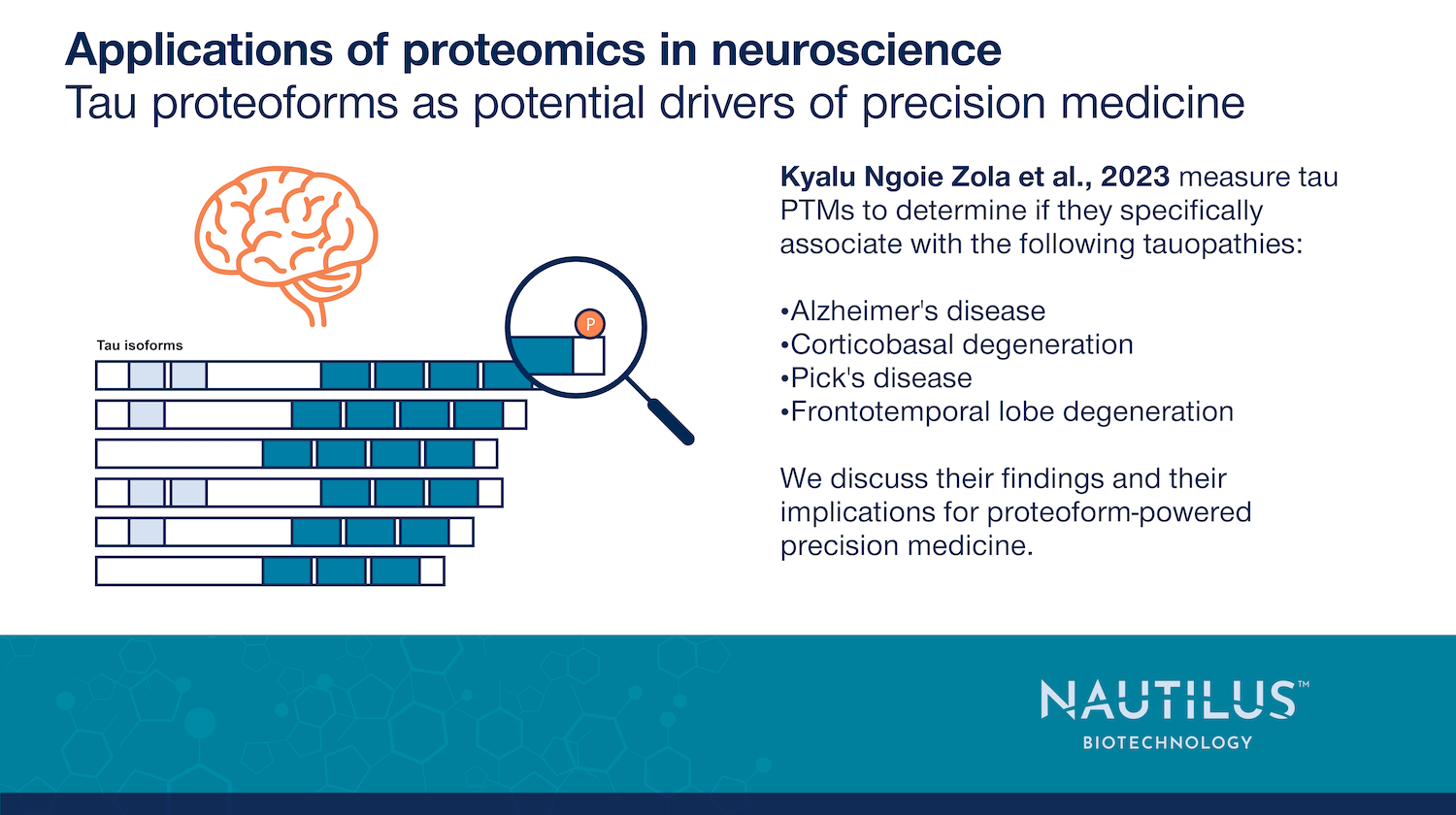
Tau proteoforms as potential drivers of precision medicine
Diagnosing Alzheimer’s disease remains a challenge. Traditionally, it was confirmed only through the identification of amyloid plaques and tau tangles in postmortem brain samples. Today, PET imaging and blood biomarkers offer earlier detection, but questions remain about their specificity—particularly for tau, which is implicated in multiple neurodegenerative disorders (tauopathies).
To improve diagnosis and treatment, researchers are investigating tau at the molecular level. Proteoforms—distinct versions of proteins created by mutations, splicing, and post-translational modifications (PTMs)—could serve as disease-specific biomarkers. Recent findings suggest that PTM patterns on soluble tau may hold the key to distinguishing tauopathies.
Kyalu Ngoie Zola et al., 2023 identified unique tau PTM signatures across Alzheimer’s and related disorders, hinting at novel diagnostic and therapeutic targets. Learning more about the tau proteoforms these PTMs give rise to could unlock precision medicine approaches for neurodegeneration.
Amyloid-beta proteoforms and Alzheimer’s disease
Amyloid beta plaques are a hallmark of Alzheimer’s, but research reveals that amyloid beta itself exists in diverse molecular forms—proteoforms—with distinct roles in disease progression. Wildburger et al., 2017 identified many amyloid beta proteoforms in plaques. These varied in their truncations and post-translational modifications. Additional studies by Bouter et al., 2013, Beretta et al., 2024 and Kandi et al., 2023, showed different amyloid beta proteoforms may be associated with different stages of Alzheimer’s and may have differential impacts on neuronal toxicity and cognitive decline.
Current Alzheimer’s drugs target amyloid beta aggregates but slow, rather than stop, cognitive decline. A deeper understanding of amyloid beta proteoforms may pave the way for more effective therapies. Emerging proteomics technologies could provide critical insights into Alzheimer’s progression and treatment.
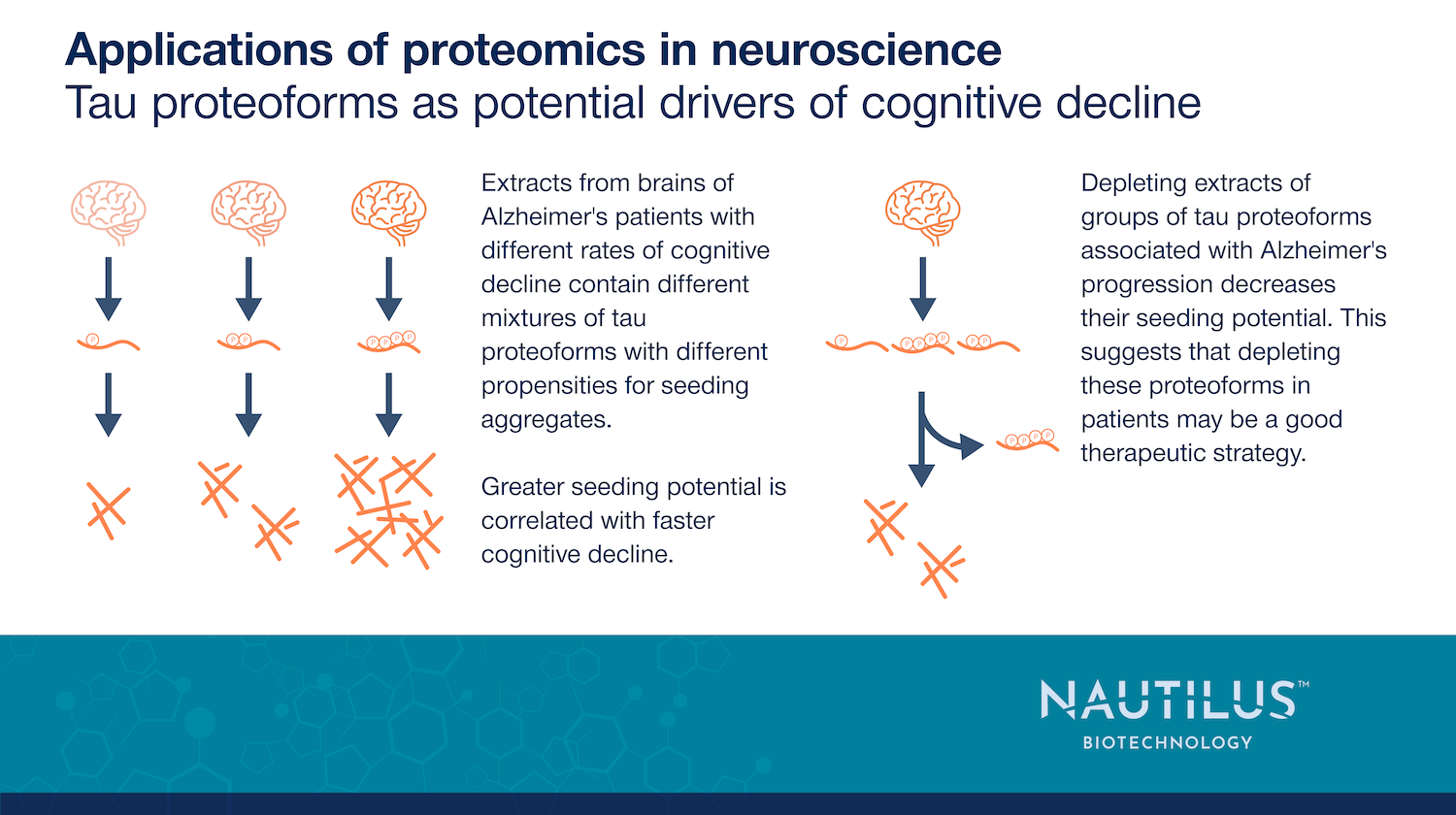
Tau proteoforms as potential drivers of cognitive decline
Tau neurofibrillary tangles are a key feature of Alzheimer’s, but not all tau molecules are the same. Tau exists as multiple proteoforms with different modifications, and recent research by Dujardin et al., 2020 suggests specific tau proteoform profiles may be linked to Alzheimer’s progression.
Brain extracts from Alzheimer’s patients with faster cognitive decline showed greater tau aggregation potential in models, correlating with higher levels of oligomeric, hyperphosphorylated, and high-molecular-weight tau. Some phosphorylation patterns promoted aggregation, while others inhibited it, highlighting the complexity of tau modifications.
Encouragingly, depleting certain tau proteoforms from brain extracts reduced their ability to seed further aggregation, suggesting a potential therapeutic strategy. With proteomics advancements, precision medicine approaches targeting a patient’s unique tau proteoform profile may become a viable treatment for Alzheimer’s.
Learn how we quantify proteoforms on the Nautilus Proteome Analysis Platform:
MORE ARTICLES