Alzheimer’s insights from amyloid beta expression in nematodes – Applications of proteomics in neuroscience

Tyler Ford
August 15, 2024
Isoforms of the amyloid beta (Aβ) protein aggregate and form insoluble clumps of protein. These “plaques” play an important role in the progressions of Alzheimer’s disease, and therapeutics designed to clear them have been approved. These promising drugs slow but do not prevent Alzheimer’s progression.
Alzheimer’s therapies may be improved through research into the processes underlying disease progression. Indeed, exactly how Aβ aggregation contributes to Alzheimer’s pathology is not understood. In their 2024 work published in GeroScience, Anderton et al., 2024 from the Buck Institute set out to elucidate more of the biology underlying this process. They knew other proteins aggregate along with Aβ in Alzheimer’s and wanted to identify which of these proteins Aβ causes to aggregate. They also wanted to determine if altering these proteins could mitigate Aβ’s effects.
Read on to learn how Anderton et al., 2024 leverage a C. elegans’ model of Aβ overproduction and proteomics to discover proteins that may play a role not just in Alzheimer’s, but many age-related diseases.
Watch this animation to learn how next-generation proteomics platforms can fuel neuroscience.
Discovery proteomics reveals age-related and Aβ-induced protein insolubility
Many human genes have orthologs in C. elegans. These nematode worms are often used in Alzheimer’s research because they are multicellular, have well-mapped nervous systems, produce many Alzheimer’s proteins, and are easier to work with than common mammalian model organisms (see Alexander et al., 2014 for a review). Previous studies in C. elegans showed:
- Aβ over-production results in C. elegans paralysis.
- Many C. elegans proteins become insoluble as the worms age.
- These age-related insoluble proteins can exacerbate the effects of Aβ over-production.
Anderton et al., 2024 first hypothesized Aβ production could trigger age-related protein insolubility. Such triggering could potentially drive a feedback loop of insolubility and pathology.
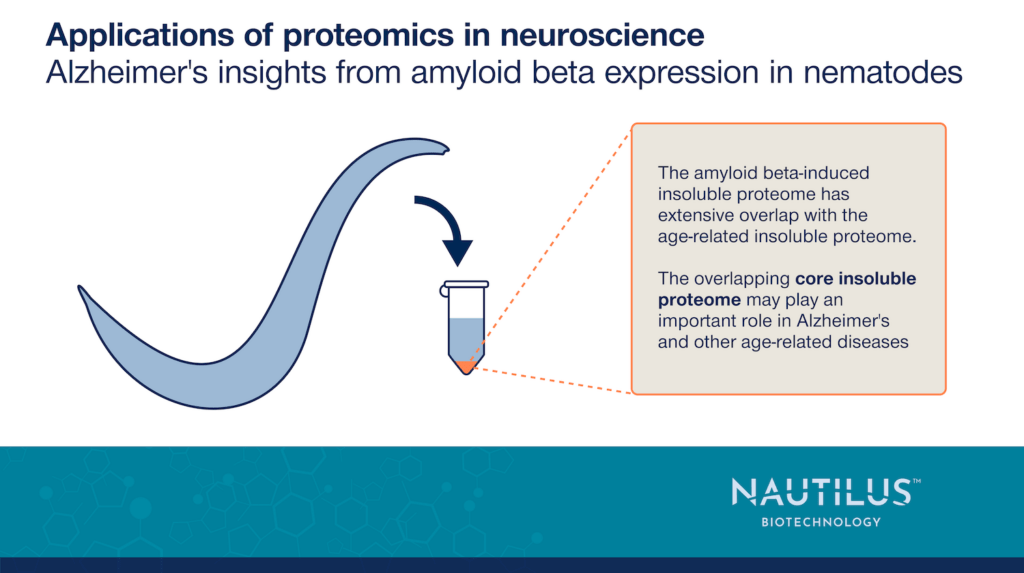
To test this hypothesis, they overproduced Aβ in C. elegans, lysed the worms, pelleted the insoluble proteins, and used discovery proteomics techniques to determine what proteins were in the pellet. They found that the number of insoluble proteins increased ~3x with Aβ overproduction. Furthermore, more than half of these proteins overlapped with those that become insoluble in aging worms. They termed these overlapping proteins the “core insoluble proteome.” Many proteins in the core insoluble proteome are associated with proteostasis, mitochondrial processes, and lifespan.
Interestingly, when Anderton et al., 2024 computationally predicted solubility, they found the average protein in the core insoluble proteome had a higher solubility score than the average protein in the proteome. Core insoluble protein expression also failed to consistently increase with age. This indicates proteins in the core insoluble proteome are not intrinsically insoluble. Instead, their insolubility may be induced by Aβ and other stimuli.
Mitigating the effects of Aβ overproduction in worms
As a proxy measure for potential impacts on disease, Anderton et al., 2024 tested if they could mitigate Aβ-induced paralysis by modulating the core insoluble proteome. They knocked down 23 members of the core insoluble proteome using RNAi and found that knock down sometimes delayed and sometimes advanced paralysis. This makes it clear that modulating the core insoluble proteome can alter the effects of Aβ, albeit in complex and sometimes opposing ways.
Given that many proteins in the core insoluble proteome are associated with mitochondrial function, Anderton et al., 2024 went on to determine if safeguarding mitochondrial function by enhancing mitophagy could delay Aβ-induced paralysis. They treated Aβ overproducing worms with Urolithin A, a drug that promotes mitophagy, and showed treatment reduced paralysis.
Altogether, these results support the notion that manipulating the biology underlying the core insoluble proteome can protect against Aβ. In addition, manipulating the core insoluble proteome may be a productive route to treating Alzheimer’s disease.
An “encompassing strategy” for treating age-related diseases like Alzheimer’s
Aβ plaque formation is a key feature of Alzheimer’s, but Anderton et al., 2024 showed proteins in the core insoluble proteome may be involved in a wide range of diseases. Indeed, these proteins are enriched for processes associated with many broad categories of age-related diseases like cancer and metabolic disorders. Importantly, the core insoluble proteome is more enriched for functions associated with age-related diseases than non-age-related diseases.
Given these associations, Anderton et al., 2024 posited that targeting the core insoluble proteome may help treat many age-related diseases. Determining the specific ways to target these proteins requires much further work, but these results point to a potential broad “encompassing strategy” for drug development.
This work could have far-reaching impacts on the ways researchers think about treating a wide variety of ailments. Anderton et al., 2024 thus provide a great demonstration of the power of proteomics supported by animal models in which physiologically meaningful hypotheses can be tested quickly. We hope the NautilusTMProteome Analysis Platform will accelerate similar research endeavors and aid the development of novel treatments for age-related diseases and much more.
Find more applications of proteomics in neuroscience in our Proteomics and neuroscience eBook.
MORE ARTICLES