Applications of proteomics in cancer – assessing the potential of PROTACs

Tyler Ford
November 28, 2023
Researchers have discovered many proteins that enable cancer cells to proliferate. Unfortunately, these proteins often have important roles to play in healthy cells. Thus, targeting them with drugs that inhibit their activity or knock down their expression can lead to detrimental side effects for patients.
Recognizing this issue, researchers have begun developing means of selectively targeting therapeutics or therapeutic activity to cancer cells. One elegant way they do so is through protein degraders such as PROTACs.
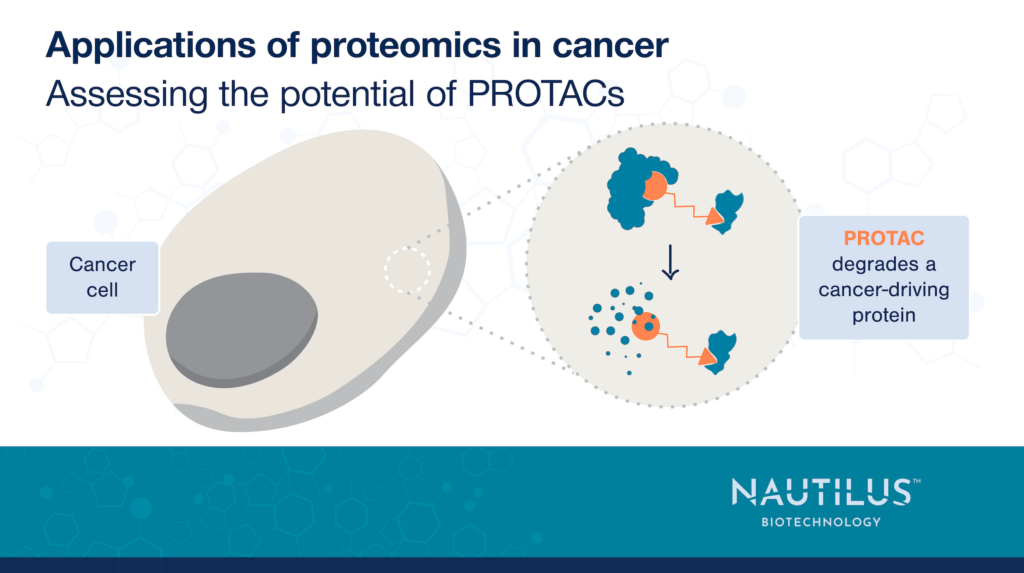
PROTACs or “proteolysis-targeting chimeras” recruit ubiquitin ligases to a protein of interest. They consist of three parts:
- A target-binding piece
- A ubiquitin ligase-binding piece
- A linker that ties the two pieces together
When target and ligase are in the same cell, the PROTAC brings them together and the ligase ubiquitinates the target. This marks the target for proteolysis by the ubiquitin-proteasome system ultimately leading to the target’s degradation.
Critically, different ubiquitin ligases are expressed in different cells. Thus, with knowledge of protein expression across cancer cells and healthy cells, researchers can design a PROTAC that degrades a target protein selectively in cancer cells.
There are many protein degraders in clinical trials, and below we cover just one example of PROTAC development. As you’ll learn, next-generation proteomics technologies have a powerful role to play in the development of these promising therapeutics.
View our animation to see how next-generation proteomics can fuel cancer research
Selective PROTACs in action
Khan et al. demonstrated the potential for tumor selective PROTACs in a 2019 publication in Nature Medicine and recently conducted a clinical trial based on their work. They designed a PROTAC that selectively kills leukemia cells by tethering the anti-apoptotic protein BCL-XL to the Von Hippel Lindau (VHL) E3 ligase. BCL-XL has long been a drug target in leukemia, but small molecule drugs against it also inhibit its activity in healthy platelets leading to thrombocytopenia, a dangerously low platelet count.
As the VHL protein is not abundant in platelets, the BCL-XL-VHL PROTAC has minimal effects on them but effectively kills leukemia cells. The researchers demonstrated this in both cultured cells and mouse xenograft models. The authors go on to show that their PROTAC can be combined with other antitumor drugs and chemotherapy regimens to treat cancer more effectively in mouse xenograft models of leukemia.
Although these findings alone are promising, the authors also leveraged mass spectrometry-based proteomics to show proof of the designed mechanism of action by demonstrating ubiquitination of a specific lysine residue of BCL-XL. They also leveraged proteomics to show that their PROTAC only reduced BCL-XL levels and not those of any other proteins. This is a promising finding because it indicates the PROTAC should have minimal off-target effects.
Interestingly, this specificity was not demonstrated in vitro where the PROTAC was able to bind to other BCL proteins. These results show that it is crucial to assess functional activity in cells and not just binding of proteins in simple, defined systems. Proteomics techniques help researchers get a comprehensive view of drug activity in live cells where the milieu is complex and dynamic. All in all, these results reveal the incredible potential of PROTACs as cancer therapeutics as well as the need to evaluate their mechanism of action carefully.
PROTAC-based treatments require a comprehensive understanding of the proteome
Khan et al. 2019’s work shows how knowledge of gene expression and the proteome can guide the creative development of new and promising therapeutics. To create an effective PROTAC, these researchers needed to know where their target was expressed, where the ligase was expressed, whether their PROTAC impacted other proteins, and how to design a PROTAC to interact with the appropriate target and ligase in the first place.
Next-generation proteomics technologies can help future researchers rapidly assess all these questions comprehensively and efficiently without the need for expertise in complex mass spectrometry workflows. This will hopefully lead to more effective treatments. Indeed, we hope that researchers will be able to use the NautilusTM Proteome Analysis Platform in similar ways to impact not just cancer but a wide range of diseases.
For more on the potential for proteomics in drug development, check out our blog post titled, “Using proteomics to improve the drug development process.”
MORE ARTICLES