Applications of proteomics in cancer – Exploring the relationship between senescence and tumor clearance

Tyler Ford
July 16, 2024
Senescence is a cellular state in which cells stop dividing and begin sending out a variety of signals. Cancer cells can enter a senescent state during tumor growth. Sometimes this promotes tumor clearance. Other times, senescent cells persist after therapy and then promote relapse.
To enhance our knowledge of the mechanisms underlying senescence and its outcomes, Chen et al. 2023 from the Lowe Lab at Memorial Sloan Kettering leveraged a variety of tools including inducible mouse models, transcriptomics, and proteomics. They discovered pathways that may be important for promoting senescent tumor clearance by the immune system. Their work has promising implications for senescence-leveraging treatments.
Check out this animation to learn how proteomics can fuel cancer research.
An inducible mouse model of senescence
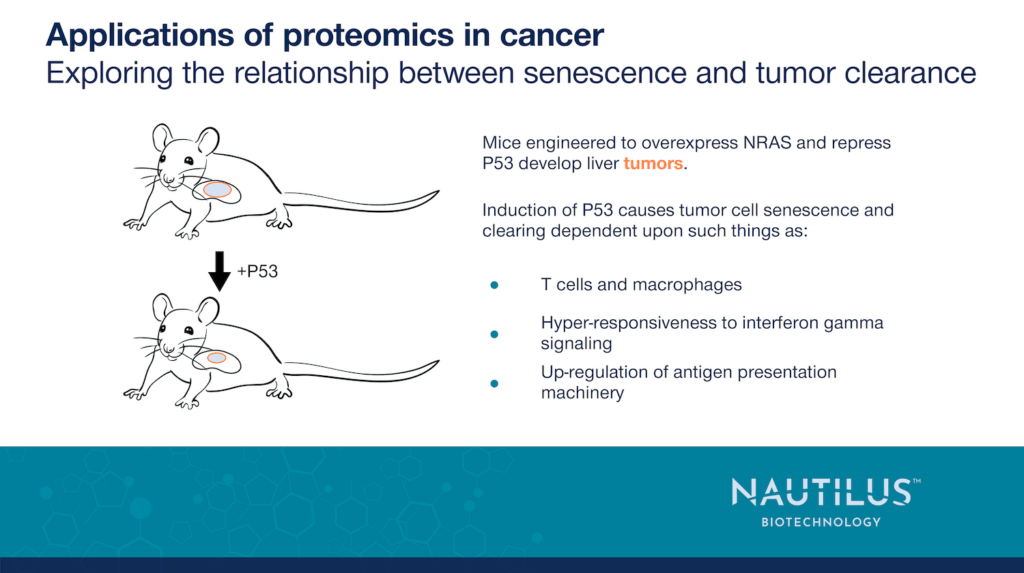
Overexpression of the NRAS protein leads to liver tumor formation in mice while activating P53 causes these tumors to senesce and regress. Knowing this, Chen et al. 2023 used an injectable transposase system that drives NRAS overexpression and enables P53 to be turned on or off. Upon injection, P53 begins in the off state, and NRAS overexpression causes mice to develop liver tumors that subsequently regress when P53 is turned on.
By transporting these tumors into mice with defects in their immune systems and inducing P53, Chen et al. 2023 show that adaptive immunity is essential for tumor clearance and the innate immune system aids in tumor regression. Further mouse work shows that subsets of T cells and macrophages are associated with senescence-induced tumor regression. Eliminating these subsets prevents regression. This work establishes a clear connection between the immune system and senescence-enabled tumor clearance in mice.
Transcriptomic and proteomic profiling of senescent cells highlights the role of interferon gamma in tumor clearance
To determine how senescent cells interact with and recruit immune cells to clear tumors, Chen et al. 2023 compared the transcriptomes and cell-surface proteomes of senescent and proliferating tumor cells. Transcriptomic analysis revealed senescent tumors had high expression of many immune-activating genes and low expression of immune dysfunction genes. Similarly, proteomic analysis of cell-surface proteins revealed up regulation of a variety of proteins involved in environmental sensing, growth factor signaling, and interactions with the immune system. Some of these surface proteins did not show changes at the transcript level highlighting the importance of proteomic analysis in this kind of work. Overall, these results point to a strong role for immune activation in senescent tumors.
One of the highly up-regulated proteins was IFNGR1 (a component of the interferon gamma receptor), and the authors go on to discover a prominent role for interferon gamma signaling in senescence. Indeed, additional gene ontology analysis revealed that many components of Type II interferon gamma response were up regulated during senescence. Chen et al. 2023 also analyzed data from other senescence studies and discovered that the Type II interferon gamma response was up regulated in a variety of cancer types. This points to type II interferon gamma response as a key component of senescence.
Further work showed that senescent cells were particularly sensitive to interferon gamma treatment. When treated with exogenous interferon gamma, these cells had high activation of downstream pathways such as JAK-STAT which is involved in many immune functions. Furthermore, senescent cells generally had upregulation of the antigen presentation machinery, and certain subsets of that machinery were also hypersensitive to interferon gamma. This may make senescent cells uniquely suited to trigger immune cells to destroy tumor cells displaying presented antigens. This effect was not recapitulated in proliferating tumor cells over-expressing the interferon gamma receptor alone. This indicates that effects downstream of interferon gamma are the result of many complex changes occurring in senescence. They are not due to one protein.
Mouse and cell models further confirm the importance of interferon gamma in senescence and tumor clearance
Using mouse and cell culture models of senescence, Chen et al. 2023 did much additional work to show:
- Senescent tumors have up regulated interferon gamma signaling in vivo compared to proliferating tumors.
- T cells and macrophages produce the interferon gamma that activates this signaling.
- Tumor clearance is impaired when senescent cells don’t express the interferon gamma receptor, or mice receiving the tumors don’t express interferon gamma.
Insights into senescence biology with potential clinical applications
These results provide evidence that interferon gamma signaling plays a key role in the immune destruction of senescent tumors. They point to a model in which:
- P53-induced senescence signaling recruits immune cells.
- The immune cells produce interferon gamma.
- Senescent cells are hyperresponsive to interferon gamma and effectively up regulate their antigen presentation machinery as a result.
- Senescent cells display tumor antigens to immune cells.
- The immune cells recognize the antigens on tumors and destroy them.
There may be different senescent responses in different tissues/cancers, the interferon gamma pathway is not solely responsible for these effects, and these results should be confirmed in additional models as well as human samples. Nonetheless, it is promising that Chen et al. 2023 found up regulation of similar pathways in other senescent cancer cells
Critically, this model depends upon the senescence phenotype triggered by P53. Many tumors have mutated P53 and it will be interesting to see if researchers can leverage the downstream biology observed here to nonetheless trigger a senescent phenotype amenable to immune destruction in P53 mutant tumors. Doing so may enable development of novel therapies effectively targeting otherwise intractable cancers.
Next-generation proteomics platforms are poised to make it much easier to see what pathways are active in senescent cells. Technologies like the NautilusTM Proteome Analysis Platform may thus make it easier to target these intricate pathways for effective treatments.
Check out this animation to learn more about the potential for proteomics to fuel cancer research.
MORE ARTICLES