
Using proteomics to personalize medicine across the continuum of care (Part 2)

Tyler Ford
July 11, 2023
For many years we’ve been told that a wave of precision and personalized medicines based on the intricate, molecular details of patient biology is just over the horizon. Unfortunately, except for niche cases like drugs targeting specific mutations in cancer (Morash et al 2018) or genome editing for cancer and rare diseases, this wave has not come.
Biologists have long sought technologies that will make personalized medicine possible and many hoped that advances in genetics and genomics were the key to unlocking this new era. Indeed, new DNA sequencing technologies have revealed genes associated with disease, and full genome sequencing can tell individuals about their potential susceptibility to certain ailments. Yet, genomes do little to inform people about their current health or if their lifestyle choices have increased or decreased their disease risk.
To truly achieve personalized medicine, we need to look beyond DNA and instead focus on the molecular machines that scientists have long recognized as fundamental units of biological activity. We need to look to proteins.
This is the second post in this two-part series and covers how next-generation proteomics technologies capable of measuring the full collection of proteins in a biological sample (the proteome) can spur the development of effective, personalized medicines and forecast future changes in patient health.
See the first post in this series to learn how next-generation proteomics technologies can establish personalized baselines of health for individual patients and help physicians diagnose disease.
Want to read the full white paper now?
Using proteomics to spur the development of effective treatments
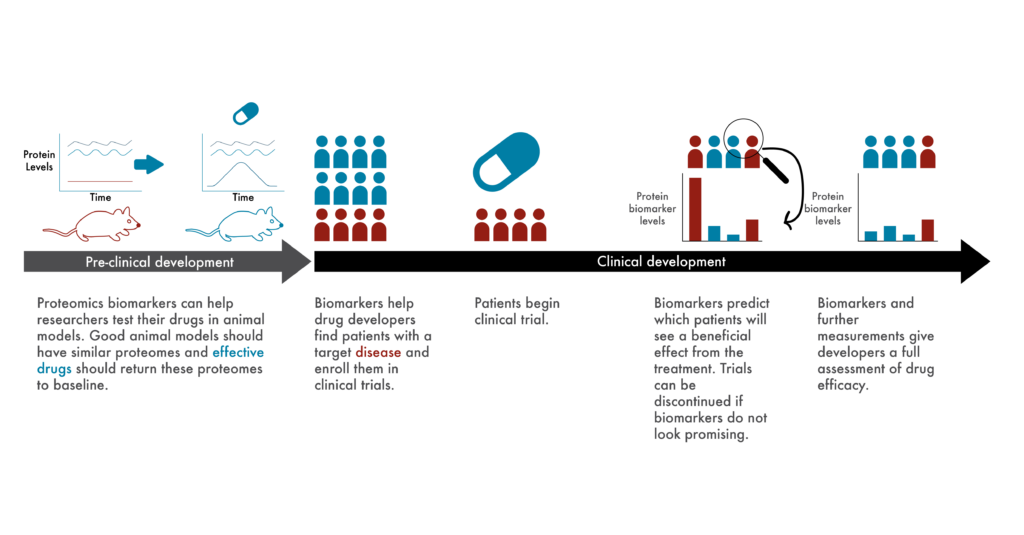
Clinical researchers spend much of their time establishing objective measures of effective treatments. From experiments in cells growing in petri dishes, to experiments in animals, to clinical trials, they need means of measuring whether experimental drugs actually (and preferably specifically) treat or cure their target diseases.
Given the differences between cells, animal models, and humans in the real world, it can be very difficult to choose metrics that confidently predict success across all levels of drug development. This is part of the reason why only ~13% of drugs make it through clinical trials (Wong et al 2019).
To solve this problem, many researchers are developing experimental systems that better mimic human biology (Walsh et al 2017), but proteomics offers a complementary solution. Rather than look at potentially superficial phenotypes associated with disease, researchers can use proteomes as objective biological measures of disease, as “biomarkers.”
For example, if a particular change in the proteome is known to be associated with heart disease, researchers could look to see if this change is copied across their various experimental systems. Given the intricate, molecular association between the change in the proteome and the disease, it’s likely that at least some of the proteins involved would have something to do with the mechanism of the disease. If a treatment returned the proteome to normal across experimental models, researchers could have more confidence that it would do so in patients.
After approval, physicians could use proteomics to continue to monitor treatment efficacy. If physicians failed to see a patient’s proteome begin to return to normal, they could take action and alter the treatment quickly.
For example, if a patient gets a bacterial infection, they may be prescribed an antibiotic. Unfortunately, this selects for the growth of other bacteria that are resistant to the antibiotic and is a particular issue with people who suffer from chronic infections such as those with cystic fibrosis (Hahn et al 2018, Scialo et al 2021). Proteomics could give physicians a leg-up on the bacteria by enabling them to monitor patient samples for the presence of proteins indicative of antibiotic resistance. Before these bacteria accumulate in the patient, physicians could prescribe new antibiotics and stop the resistant bacteria in their tracks.
Using proteomics to forecast changes in patient health
Even if a patient doesn’t feel sick yet, changes in their baseline proteome could alert physicians to future problems. This would help physicians prevent the development of a serious illness.
For example, if a patient has a family history of heart disease, physicians could pay special attention to protein changes associated with their heart health. Physicians might begin to see changes in these proteins before something catastrophic like a heart attack occurred and could act on these early warning signs. Given that over 650,000 people die of heart disease annually in the US alone, physicians could potentially save hundreds of thousands of lives by pre-emptively putting at-risk patients on preventive heart medications in this way.
Similarly, proteomics measurements can complement the disease susceptibility information provided by genomics. Genetic information may altert a physician to a patient’s pre-disposition for heart disease. This physician could then pay more attention to changes in the patients’ proteome associated with heart health and prescribe preventative measures as soon as they see things begin to deviate from normal.
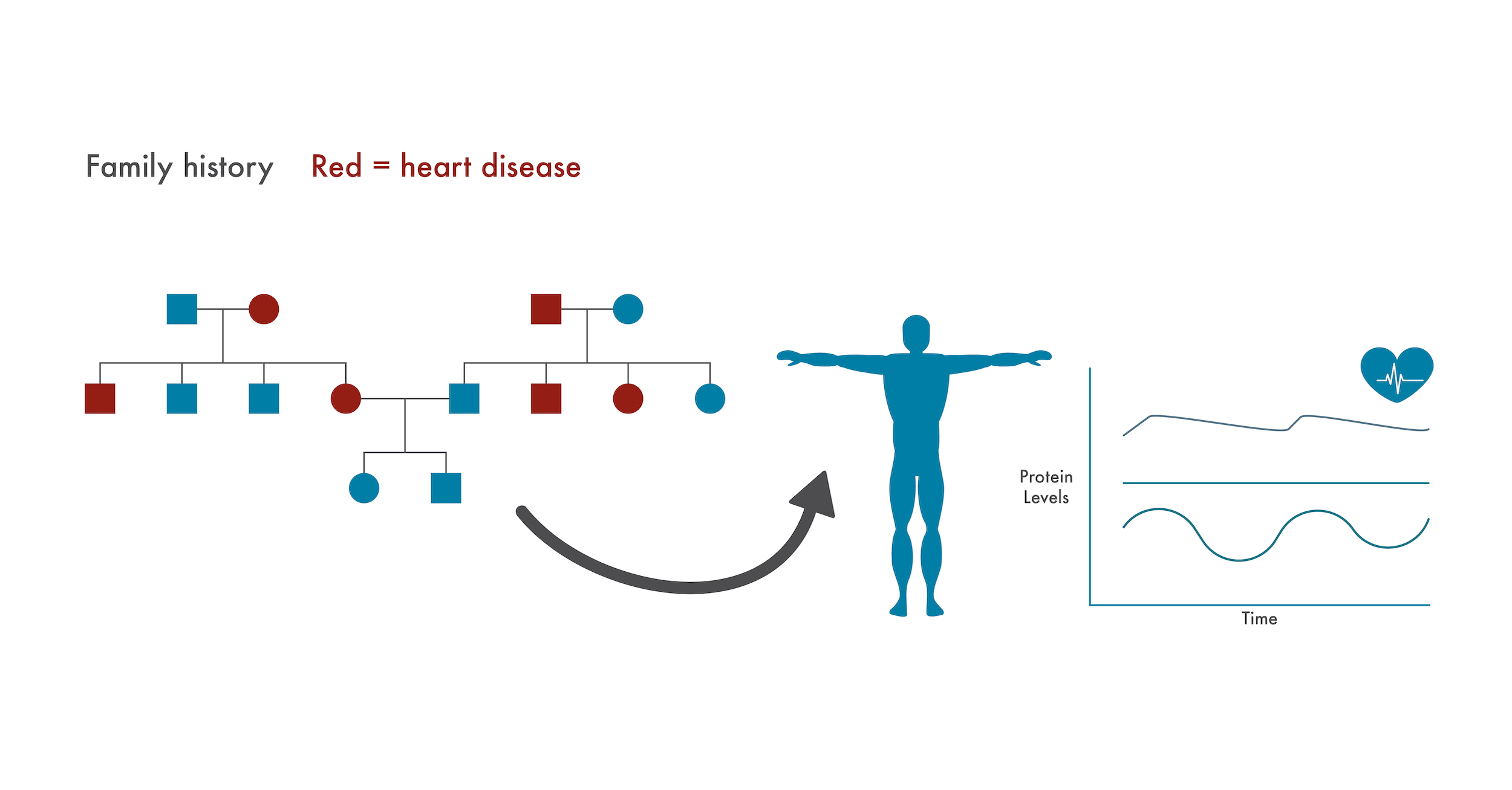
If a patient has a family history of a disease with a genetic component (heart disease here), caregivers can specifically monitor changes to the proteome associated with that disease. Doing so, they may identify issues and prescribe preventative treatments before serious health issues arise.
The proteomic gold standard in precision medicine
Proteomics will soon become the gold standard for technologies that make personalized medicine possible for all. Through proteomics, we can establish critical, personalized baseline measures of patient health and associate changes from this baseline with the molecular causes of sickness. This will make it much easier to diagnose ailments, prescribe appropriate treatments, and develop new therapeutics. Advances in proteomics will finally give biologists and physicians the tools they need to probe the depths of protein biology in near real time and make the long-hoped-for era of personalized medicine a reality.
Want to read the full white paper now? Download “Using proteomics to personalize medicine across the continuum of care” here.
MORE ARTICLES
